Rhodium - (l) [ Rhcl( PPh₃) is called wikinson's catalyst. It's discovered by wikinson's in 1965.The red voilet colored compound is prepared by refluxing ethanoic acid. Rhcl₃.3H₂O with an excess of PPh₃. This is a 11 electron d⁸ species. It has a square planner geometry.
It has effective homogeneous catalyst, it's remarkably affective because hydrogenation of isolated double bond (or) triple bond takes place at atmospheric pressure and at room temperature.
It's, application in pharmaceutical industries for making specific drugs in spite of the high cost of the catalyst.
HYDROGENATION OF FUMARIC ACID:
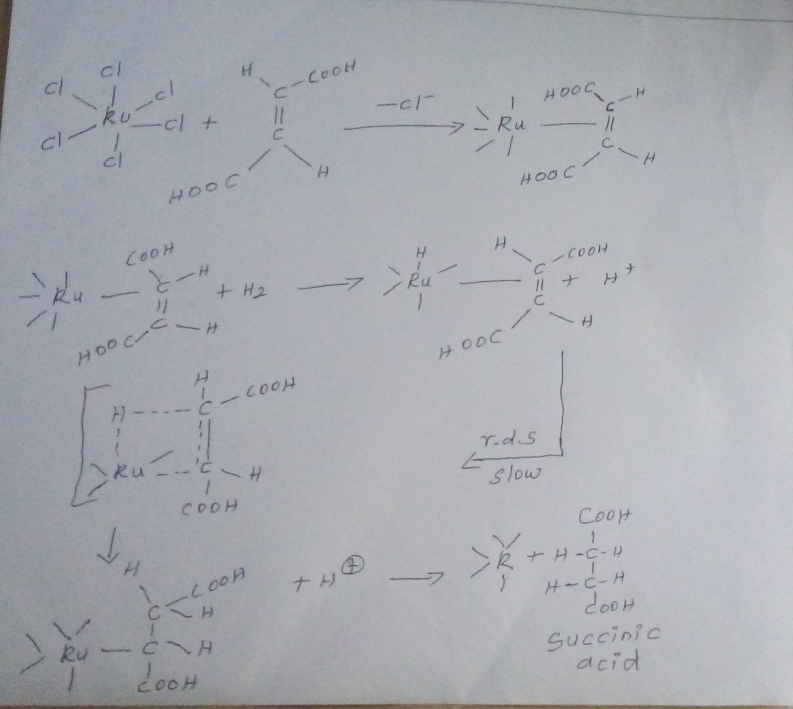
Ruthium (ll) chloride complex act as a catalyst for hydrogenation of maleic acid or fumaric acid. The metal chloride forms as 1:1 metal- olefin (ll) - complex
In maleic and fumaric acid, hydrogenation occurs in a slow step at 60-90⁰c because they contain activated double bond - Due to the presence of a carboxylic group the total net reaction is
Mechanism :_
Reaction proceed through four center intermediate first a ruthenium alkyl compound is formed. Then electrophilic attack of H+ ions takes place on the Ru-c, sigma bond with restriction of configuration.




Very nice
ReplyDelete