After sometime, the electrolysis stops as current is not passing through the solution.
Under these conditions, Fe has become inactive or inert or noble or passive.
So passivity is an anodic phenomenon. It's an irreversible, process at the anode.
TYPES OF PASSIVITY :
Type 1: Mechanical Passivity :
It's due to coating of metal surface with multiple and visible oxide layer.
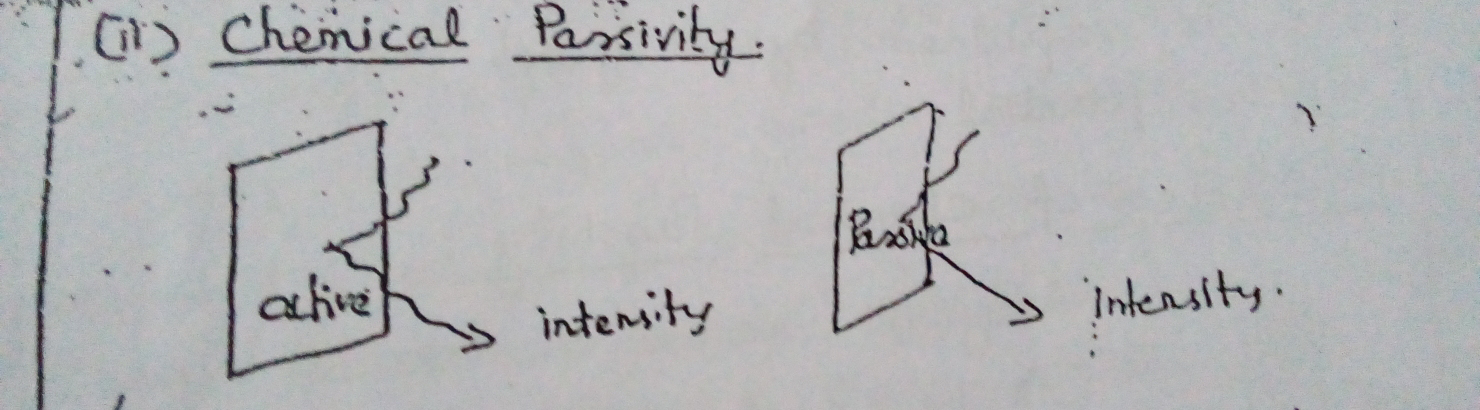
Type 2: Chemical Passivity :
This is due to coating of metal surface with invisible unimolecular oxide layer.
During the electrolysis of solutions with Fe anode, at the anode Fe dissolves forming Fe²⁺ At the same time OH⁻ deposits at the anode forming oxygen gas. The Fe²⁺ with OH⁻ can form Fe ( OH)₂ At higher anodic potentials in the presence of oxygen, Fe ( OH)₃ will be formed and by the dehydration of hydroxides, the respective oxides will be formed. These oxides get coated at the anodic surface.
Due to coating an anodic surface with oxide layer, the anodic SA decreases.
As current density (CD) =
Current
Surface area (SA)
When SA decreases > Current density will increase.
With increasing applied potential, CD increase and attains a maximum with SA is minimum.
Once anodic surface is completely coated with the oxide layer, electrolysis steps as OH- cannot get deposited at the anodic surface. Then CD drops to a minimum. When the electrode has become passive. On lowering the potential, CD remains to be a minimum and never traces the same path. Hence the process is irreversible. This is explained by a graph of CD versus applied potential.
By ||| ʳ method an oxide layer will be coated at the metal surface because it's porous, OH- ion can reach the anode through the holes and get deposited. Then O₂ gas fully coated, pressure all the oxide layers except the first are below up. The first layer is strongly attached to metal surface by chemical forces. (Chemisorption) Hence it's retained.
Evidence for the mechanism :
1) Passivation depends upon the PH of medium.
The intensity of scattered radiation by the chemically passive metal is different from that of active metal. This shows that the metal surface has undergone a definite change.
3) Chemical Passivity :
The chemically passive metal is introduced in around and the metal is dissolved learning behind a then oxide film.
Methods of making passive metal active :
1) Using an energy paper, the oxide layer can be removed from the passive metal.
2) The passive metal is made as cathode. When H+ deposit on metal oxide, it's reduced to metal or metal becomes active.
Uses of Passivity :
1) In the construction of lead oxide accumulate Pb is coated with PbO₂ solid by keeping Pb a anode during electrolysis. This is known as anodic process.
2) By coating with metal oxide on a metal the surface becomes, coloured and beautiful. Hence it can be used for decoration purpose namely in making flower rose.








No comments:
Post a Comment
Thanks for reading